TMS therapy uses a computerized, electromechanical medical device to produce and deliver non-invasive, magnetic stimulation using brief duration, rapidly alternating, or pulsed, magnetic fields to induce electrical currents directed at spatially discrete regions of the cerebral cortex. This method of cortical stimulation by application of brief magnetic pulses to the head is known as transcranial magnetic stimulation or TMS. When pulses of TMS are delivered repetitively, this is called repetitive TMS, or rTMS. These pulses can be delivered at either high (10–20 Hz) or low frequency (less than or equal to 1 Hz). Most clinical TMS treatments delivered for treating depression are typically given at 10 Hz to 18 Hz. The peak magnetic field strength achieved with each pulse is approximately 1.5 Tesla, right underneath the coil, similar in strength to the magnetic field produced by a typical magnetic resonance imaging (MRI) device. The MRI field is large (filling much of the room) and is constantly on. TMS magnetic fields are focal and brief. In 2008, the United States Food and Drug Administration (FDA) cleared the first TMS device for therapeutic clinical use in Major Depressive Disorder (MDD). This device was a focal iron core coil produced by Neuronetics Inc. (Malvern, PA, USA). In 2013, the FDA cleared a second device (i.e. the H-Coil) produced by Brainsway (Jerusalem, Israel). In 2015, two additional devices were FDA cleared, the Magstim Company’s (Wales, UK) figure eight coil and Tonica’s (Magventure) figure eight coil. Product manufacturer manuals provide technical details about each coil and system, which are beyond the scope of this review.
Dr. Perera is one of the lecturers of Columbia University’s CME course on Electroconvulsive Therapy (ECT) that trains psychiatrists from around the world on the basic principles of ECT Therapy. He worked with Dr. Harold Sackeim’s team to develop a novel ECT treatment protocol that had less side effects.
Dr. Perera discusses the differences between mood disorders (such as depression and bipolar disorder) and personality disorders (eg. Borderline Personality Disorder). Mood disorders with affective lability constitute a cluster of syndromes with many overlapping factors. Among these, Borderline Personality Disorder (BPD) and Bipolar II (BPII) are the most prevalent.
Mood lability is a familial trait, hence many patients will have parents who are moody as well. This could lead to emotionally absent parenting during the key moments of childhood ego development. The result will be ego impairments that include basing one’s self-worth based on how others treat you. Depending on others for one’s happiness leads to fears of abandonment which is a core feature of Borderline Personality Disorder. If however, there is normal ego development during childhood, the person will have a healthy ego but nevertheless be subject to the familial trait of mood lability that can present as moody depression or Bipolar II Disorder without borderline features.
As we shall see in the model described below this description from 1917 was amazingly prescient.
Without the aid of contextual memory, animals cannot make informed decisions in dealing with problematic situations, and this lack of coping capability would lead to hopelessness. Continued exposure to such hopelessly negative conditions would gradually turn positive memory into a negative one, even without the presence of new neurons to learn the new negative stimuli. This is probably so because mature neurons, to a limited extent, can be recruited to learn novelty as previously discussed. Learning novelty by mature neurons, however, comes at the cost of wearing out the old memory. As mature neurons become more negatively conditioned and there is no neurogenesis, the situation becomes particularly alarming. Negative memory would start acting as a form of stress in and of itself. It is as if the hippocampus is short-circuited for glucocorticoid by the dominantly negative memory fostering the hyperactivity of the HPA axis.
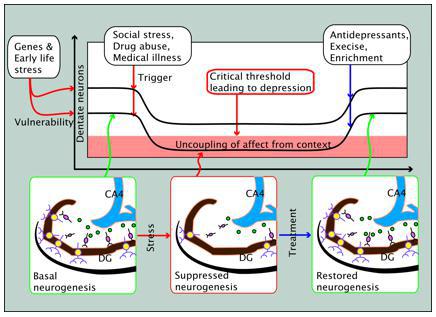
In acute stress conditions, halted neurogenesis could be beneficial for two reasons. One is that it fosters a fast learning curve by the reserve of immature neurons. Another is that excessive formation of negative memory can be retarded. In chronic conditions, however, lack of neurogenesis would be disadvantageous since it would not only disallow learning new context in the long run but also leave mature neurons vulnerable to become negatively conditioned. A depressed patient whose neurogenesis is arrested and memory dominantly negative would have difficulty incorporating positive experience into memory, and by the same token simple restoration of neurogenesis without subjecting the patient to positive experience would not help treat the patient. For an antidepressant to be effective, restoration of the normal neurogenesis rate would have to be accompanied by positive experience. The memory hypothesis gives viable explanations why halted neurogenesis per se is not a sufficient cause of depression, as well as why restoration of neurogenesis alone is not sufficient for the treatment of depression.

Click here to listen to a “Chamber Chat” event with Dr. Tarique Perera of Danbury’s leading psychiatry center, Contemporary Care. Hear directly from Dr. Perera as to how they are navigating the COVID-19 environment in support of our community.